
For scientists who perform medical research on the recently deceased, there are few regulatory or ethical guardrails.

By Jyoti Madhusoodanan
Senior Contributor and Science Writer
Undark Magazine
When the Philadelphia-based company Bioquark announced a plan in 2016 to regenerate neurons in brain-dead people, their proposal elicited skepticism and backlash. Researchers questioned the scientific merits of the planned study, which sought to inject stem cells and other materials into recently deceased subjects. Ethicists said it bordered on quackery and would exploit grieving families.
Bioquark has since folded. But quietly, a physician who was involved in the controversial proposal, Himanshu Bansal, has continued the research. Bansal recently told Undark that he has been conducting work funded by him and his research team at a private hospital in Rudrapur, India, experimenting mostly with young adults who have succumbed to traffic accidents. He said he has data for 20 subjects for the first phase of the study and 11 for the second — some of whom showed glimmers of renewed electrical activity — and he plans to expand the study to include several more. Bansal said he has submitted his results to peer-reviewed journals over the past several years but has yet to find one that would publish them.
Bansal may be among the more controversial figures conducting research with people who have been declared brain dead, but not by any stretch is he the only one. In recent years, high-profile experiments implanting non-human organs into human bodies, a procedure known as xenotransplantation, have fueled rising interest in using brain-dead subjects to study procedures that are too risky to perform on living people. With the support of a ventilator and other equipment, a person’s heart, kidneys, immune system, and other body parts can function for days, sometimes weeks or more, after brain death. For researchers who seek to understand drug delivery, organ transplantation, and other complexities of human physiology, these bodies can provide a more faithful simulacrum of a living human being than could be achieved with animals or lab-grown cells and tissues.
But surging interest in xenotransplantation, predicted by some estimates to be a $25-billion-dollar industry within the next five years, has also elicited questions about the challenges these studies can pose to families and to the broader health care system. And it has renewed concerns about regulatory loopholes that in many countries allow those studies to be conducted with little oversight.
“The rights that attach to a living human being are no longer there,” said bioethicist Brendan Parent of New York University, who noted that in the U.S. and other countries, studies with brain dead subjects are beholden neither to the regulations that govern research with living human subjects nor to those that guide work with extracted human cells and tissue.
It is a gap that has allowed physicians like Bansal to pursue, with few safeguards for subjects, research that many experts view as ethically fraught. The lack of uniform guidance has also complicated matters for research groups who seek ethics approvals but are unsure how, or where, to obtain them.
Many experts now say the time has come to replace the current patchwork of guidance with a more robust system of oversight. “The dead are voiceless,” Parent said. “There’s no one really there to speak up except for family.”
The notion of studying risky medical procedures in people who have been declared brain dead is believed to have first cropped up in the early 1970s, shortly after the first modern ventilators entered clinics. But it was in the 1980s that the idea started to gain traction with scientists, according to Parent. In early 2000, Rebecca Pentz, then a clinical ethicist at the University of Texas MD Anderson Cancer Center, was approached by researchers at the center who wanted to study whether a class of viruses known as bacteriophages could deliver drugs to tumors. The researchers’ plan called for a grueling series of procedures — 15 biopsies on a single patient over a span of 24 hours — to track where the viruses wound up in the body. “You can’t do that in a living human being,” Pentz, now a professor at Emory University School of Medicine, recently recalled. “That’s too aggressive.”
Pentz and other ethicists worked with the researchers to come up with alternatives, and they hit upon the idea to enroll people who were recently, or soon to be, deceased. Then, as now, federal regulations offered little guidance on how such a study should be designed. The Federal Policy for the Protection of Human Subjects, also known as the Common Rule, contains provisions that protect living people who participate in many research studies, as well as regulations that govern the use of identifiable human specimens. But the Common Rule does not apply to subjects who have been declared neurologically dead, so such studies are not under the purview of an institutional review board, or IRB, an oversight body that — had the subjects been alive — would have had a say on whether the study could proceed.
In the case of the proposed MD Anderson bacteriophage study, Pentz and her colleagues crafted their own set of ethical guidelines. Among the stipulations: The researchers would have to obtain consent from the families of the deceased before undertaking any experiments, and they would have just the 24 hours in which to work — no longer — before returning the body back to the family.
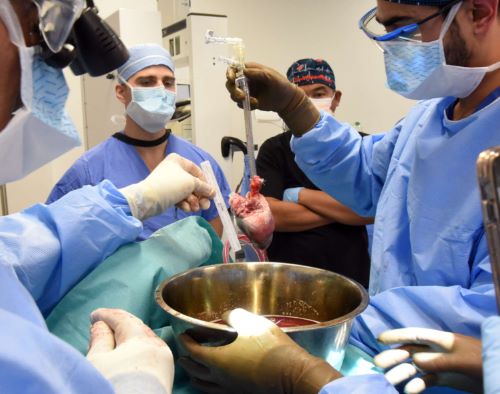
Around the same time as the MD Anderson study was getting underway, University of Pittsburgh researcher Thomas Starzl and collaborators also proposed working with brain-dead subjects. He and his colleagues were interested in exploring whether animal organs could be transplanted into humans, which they hoped could help offset a shortage of human donors. When Starzl, who passed away in 2017, and his colleagues spoke with bioethicists at the university about the idea, they recognized the need for oversight and established a campus-wide Committee for Oversight of Research and Clinical Training Involving Decedents, or CORID, which functioned much as an IRB would for human subject research.
CORID’s guidelines were broadly similar to those adopted at MD Anderson. They stated, among other things, that researchers working with the recently deceased must obtain consent ahead of time from those who would pass away, or from their next of kin; that they must ensure those families understand the scope of the study; and that a decedent’s organ-donor election must be prioritized over any research needs. “The institution wanted to be sure that we had processes, procedures, and safeguards in place really to mirror those that are in place for human subject research,” said Barbara Barnes of the University of Pittsburgh, CORID’s current chair.
Still, experts saw a need for more standardized recommendations that would guide not just researchers at a particular institution, but the field at large. In 2005, a multidisciplinary panel of experts from MD Anderson, the University of Pittsburgh, and other institutions published a broad set of ethical guidelines for working with the recently dead, a categorization that included the recently brain dead. Those guidelines remain among the handful of documents available to guide research with brain-dead patients today, and they are still frequently referenced, Pentz said.
But Pentz and other experts also suggest that informal guidelines are not a substitute for a robust system of independent review and oversight. And as xenotransplantation studies burgeon, they say the need for such a system is becoming increasingly apparent.
By definition, a person who has been declared brain dead is both legally and clinically dead: A consensus view among physicians is that once a person’s neurological activity has ceased, it is impossible for them to regain consciousness, even if their heart continues to beat. However, a brain-dead participant in a research study remains a part of society in a way that cadavers donated to medical research do not: Typically, there is an expectation that the body will be returned to their loved ones to be remembered, mourned, and laid to rest. The time spent in a research study is a pause in the social, cultural, and psychological aspects of a family’s grieving process.

The family of Alva Capuano lived through such a pause in July 2022, when she was declared brain dead after being discovered unconscious in her home. Capuano had elected to be an organ donor. But after enduring Type 1 diabetes, a kidney transplant, and complex infections over the course of her life, her body was worn out, and many of her organs were unsuitable for donation. “We got to a point where it just became very trying on us,” said Tim Capuano, Alva’s son.
Then Capuano’s family was told about an alternative: Alva could be included in a xenotransplantation study, in which physicians would place a pig heart, genetically modified to reduce the risk of rejection, into her body. The study was designed to test the heart’s ability to function normally in humans. Tim and other members of the family spoke with the study lead, NYU surgeon Robert Montgomery, who helped them feel at ease. They came to see participation in the study as an option Alva herself might have chosen. Tim remembers it being a difficult decision for the family, especially at a time when they sought emotional closure, but they decided to go forward.

Within days of Capuano’s death, Montgomery and his team transplanted the pig heart into her and began monitoring the organ’s functions, while a ventilator supported her breathing. Tim recalls that during the study, the clinicians caring for his mother called the family with daily updates. “They were pretty good about telling us throughout what was happening, how stable my mom was,” he said.
Alva was the second person to receive a pig heart in the NYU study, and the researchers continue to learn from the data they gleaned from her body’s immune response and other functions. “It’s a pretty meaningful thing,” Tim said of his mother’s contribution to science, “and something I’ll be very proud to tell my daughter about one day.”
According to Pentz, the sense of gratification Tim Capuano felt is not unusual among family members whose recently deceased loved ones participate in medical research. In interviews conducted around the MD Anderson study, she and her colleagues found that families are often grateful to give back to science in the midst of their grieving.
But ethicists also worry that families can be vulnerable to emotional and psychological harms. And although Montgomery’s team took measures to keep the Capuano family informed both before and during the study, those kinds of protections are not guaranteed, Parent, the bioethicist at NYU, said.
Plus, the potential harms may mount as researchers push to do longer, more extensive studies of the dead, experts say. The study that involved Capuano lasted only a few days, but a more recent kidney xenotransplant study, also by Montgomery’s NYU team, lasted two months. Montgomery said they needed that time to learn how to manage such transplants, and family members were given the option to pull out of the study at any time. But ethicists such as Parent and Pentz raise questions about whether there should be time limits on experiments with the dead. Studies that run weeks to months are essential to understand the infection risks posed by xenotransplants, Pentz said, but that can postpone funerals and “gets real complicated.”
Parent also noted that long studies can tie up critically important clinical resources. Ventilators, bypass machines, hospital beds, and other pieces of equipment that are used to save patients’ lives are often the same ones used to conduct studies after brain death. Nurses who contribute to those studies might, in some instances, be dividing their time between caring for the deceased and the living. That kind of competition for resources can’t be resolved solely in the context of an individual study, Parent said, but instead requires collective planning and priority-setting across the broader research enterprise.
“These things need to be well thought out ahead of time,” said Barnes, the chair of CORID at the University of Pittsburgh. “It’s obviously an urgent situation when someone might qualify for this type of research. It’s not the time to implement processes and procedures in haste.”
Even the language used to describe research with brain-dead subjects can feel slippery. Barnes prefers to describe the experimental subjects as “brain dead cadavers” to emphasize they are deceased. Pentz calls them “recently deceased persons” to indicate they are dead but still deserving of the respect we give to other humans, and are awaiting return to their loved ones. Research studies alternately refer to their subjects as donors, deceased patients, or decedents.
And then there is the matter of what to call the studies themselves. By definition, they are not clinical trials, since they do not involve living human subjects. But even the researchers who perform the experiments are sometimes uncertain what exactly to call them.
Such is the case for neurologist Rajat Dhar of Washington University in St. Louis, who has conducted human-to-human organ transplant research in brain-dead subjects for about 15 years. Although Dhar’s studies follow a similar procedure to clinical trials, he says the overarching goals are different. People who enroll in clinical trials can usually expect benefits either for themselves or for the larger community of people with the condition being studied in the trial, Dhar says, but his studies of organ function in brain-dead subjects are solely for the purpose of improving transplant rates and outcomes. There’s “a broader perspective,” he added.
Yet, when Dhar and his colleagues recently set out to do a heart transplant study involving 838 brain dead donors, they registered it in a clinical trial database anyway. “There isn’t really any other language,” he said. “We didn’t really have a framework of how we document this.” When Dhar and his team sought an IRB’s approval for that study, they were told that it involved non-human subjects and was therefore exempt from the oversight reserved for clinical trials.
Over the years, Dhar says his work has led to important changes in how human organ donors are treated shortly after death — changes that have helped improve the odds of successful lung transplants and grow the supply of available donor organs. For Pentz, the potential for social benefits like these are one reason why experiments with decedents should not be done in obscurity and shielded from oversight. She said there is a need for broad ethical guidance that prioritizes the family’s needs, ensures their consent and their understanding of the research study, and respects their grieving process. Regardless of the nature of the research, Pentz said, “the same regulations should apply.”
At the same time, experts recognize that certain studies will pose unique risks. Interventions that seek to bring someone back from brain death — as Bansal’s experiments in India are designed to do — need to be regulated differently, said Dhar. As he sees it, the goals of a study should inform the limits of those regulations.
As of now, those limits are, in many cases, undefined. Researchers like Bansal are generally free to police themselves. According to him, after media coverage drew attention to his work, he began, at times, referring to his work not as a clinical trial but as an “extended period of observation in subjects who have been declared brain dead clinically.”
Parent and other researchers find this state of affairs troubling. The lack of regulations means research on brain-dead subjects could go unchecked, Parent said. “That needs to change, in my opinion.”
Last March, Parent was among a group of researchers and ethicists who convened at the National Institutes of Health to, among other goals, discuss the importance and ethics of xenotransplantation studies. As such research and other studies with brain-dead participants forge ahead, questions of how to do the work ethically will take on new urgency, Parent said, and they should be framed by systematic guidance: “These are questions that I think we want to sort of think about and answer as a society.”
Originally published by Undark Magazine, 03.25.2024, republished with permission for educational, non-commercial purposes.


